Introduction: Hypersensitivity reactions with asparaginase occur frequently in pediatric patients (pts) with acute lymphoblastic leukemia (ALL). The standard approach for pts with reaction to E.coli-derived asparaginase is to switch to Erwinia asparaginase, given concern that clinical reactions reflect presence of neutralizing antibodies; however, Erwinia requires more frequent dosing and is often unavailable. Therapeutic drug monitoring allows for discrimination between pts with pegaspargase hypersensitivity who have sub-therapeutic asparaginase activity and those still able to derive therapeutic benefit from pegaspargase. We prospectively piloted re-challenging pts with pegaspargase after initial Grade 2 hypersensitivity to this agent, with premedication at re-challenge and assessment of serum asparaginase activity (SAA).
Methods: Pts aged 1 to < 22 years with newly diagnosed ALL were eligible for DFCI 16-001. Pts received 1 dose of intravenous pegaspargase during Induction, and every 2 weeks for 15 total doses in Post-Induction phases, without routine premedication. Pts were monitored during/after pegaspargase for allergy, with CTCAE version 4.0 event grading. Those with ≥Grade 3 allergy discontinued pegaspargase and were switched to Erwinia. Those with Grade 2 allergic reaction were eligible for pegaspargase re-challenge with pre-medication (acetaminophen, diphenhydramine, and hydrocortisone, or per institutional standard) and slower infusion rate. If < 50% of the intended dose had been administered when reaction occurred, re-challenge was within 1-7 days of initial reaction. If ≥ 50% of the intended dose had been given, re-challenge was at next planned pegaspargase dose. SAA was measured 1-hour, 7-days, and 14-days after the re-challenge infusion (if dose completed). If 1-hour or 7-day level ≥ 0.1 IU/mL, and 14-day level ≥ 0.025 IU/mL, SAA was considered adequate, and the pt continued to receive pegaspargase with premedication. Pts with an inadequate SAA level, or with new ≥ Grade 2 allergic reaction with the re-challenge dose were considered to have failed re-challenge and were changed to Erwinia (or enrolled on a clinical trial of recombinant crisantaspase, an alternative Erwinia preparation).
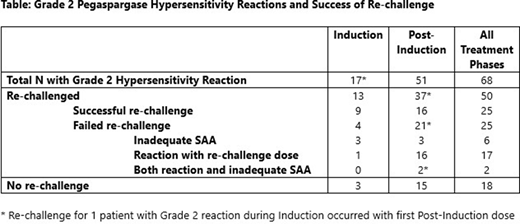
Results: Between 3/2017- 7/2020, 317 eligible pts enrolled. Overall, 81 of 299 (27%) total evaluable pts experienced a first allergic reaction to pegaspargase, 68 pts with Grade 2 reaction, 13 with Grade ≥3. During Induction, 17 of 299 (6%) evaluable pts had allergic reaction to pegaspargase; all Grade 2. Of the 17 Grade 2 reactions, 13 pts (76%) underwent re-challenge in Induction, 9 (69%) re-challenges successful and 4 failed. Post-Induction, 64 of 241 evaluable pts (27%) had a first allergic reaction; 51 Grade 2 and 13 Grade ≥3. Thirty-six of 51 (71%) pts with Grade 2 allergy during Post-Induction underwent re-challenge, as did 1 additional pt with allergy during Induction who was re-challenged with first Post-Induction pegaspargase dose (per protocol guideline, due to receiving ≥50% of Induction dose). Among these re-challenges, 16 were successful, 21 failed. Overall, 25 of 50 (50%) pts who were re-challenged after Grade 2 reaction had a successful challenge and were able to continue pegaspargase. Among the 25 pts with failed re-challenge, 6 pts (24%) had inadequate SAA alone as cause of failure, 17 pts (68%) had an allergic reaction with the re-challenge dose, and 2 (8%) additional patients had both allergic reaction and documented inadequate SAA. Three pts who were successfully re-challenged had a subsequent allergic reaction to pegaspargase. Among the 22 pts who experienced another allergic reaction with pegaspargase (at re-challenge or subsequent dose), 19 pts (86%) experienced Grade 2, and 3 pts experienced Grade 3 reaction.
Conclusion: Fifty percent of pts with a Grade 2 reaction to pegaspargase were able to tolerate and achieve adequate SAA when re-challenged with premedication. For those who did react with or after re-challenge, reactions were not more severe. The re-challenge approach limits premedication exposure only to a minority of pts with a history of prior reaction and substantially decreases the number of pts needing to switch to Erwinia asparaginase, which can be challenging to deliver due to administration schedule and drug shortage.
Place:Novartis: Consultancy, Other: Institutional Research Funding; AbbVie: Consultancy. Silverman:Takeda: Other: advisory board; Servier: Other: advisory board; Syndax: Other: advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal